
Telomere science is no longer theoretical. Understanding how cellular aging intersects with tissue repair, collagen production, and inflammation can help refine patient selection and improve long-term outcomes. Telomere dynamics also open the door to more personalized anti-aging strategies, especially when integrated with regenerative therapies and biological age assessment tools.
Educational platforms like HubMed Ed offer the Anti-Aging Regenerative Medicine Course Online and other training for professionals who want to become experts in anti-aging and cellular therapies. Integrated education like this reflects the shift away from surface-level correction toward root-cause, biology-based approaches to aesthetic longevity.
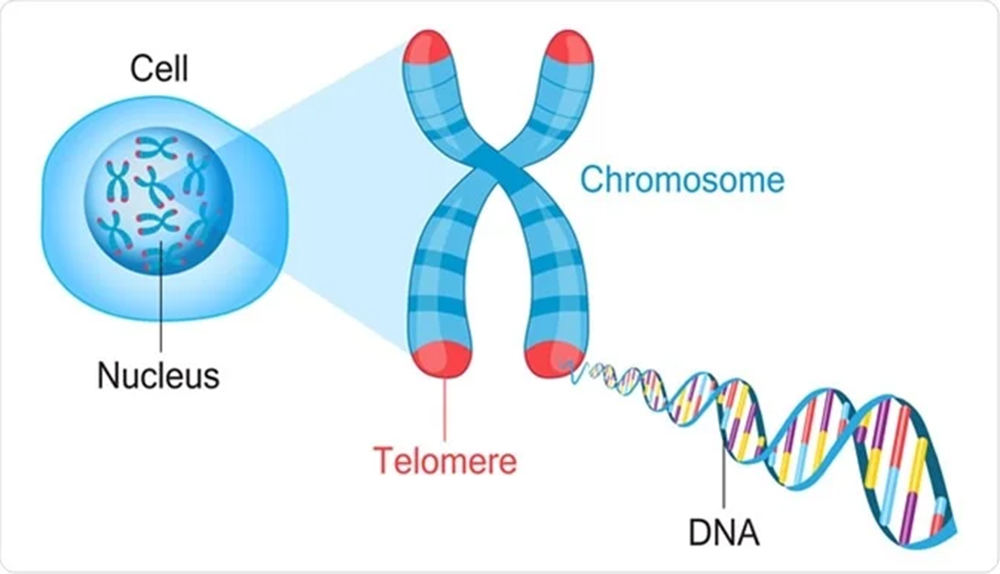
What Are Telomeres?
Telomeres are repetitive DNA-protein structures that cap the ends of linear chromosomes. Telomeres act like protective caps on the ends of chromosomes, keeping our genetic material stable during cell division. Without them, that material would slowly unravel and degrade. With each replication, these caps become shorter, eventually signaling cells to enter senescence or apoptosis.
This gradual telomere attrition is not merely a reflection of cellular wear and tear, but it is also a potent biological age marker. In aesthetic medicine, telomere length can offer insights beyond visible skin aging, indicating deeper tissue vitality and the patient’s systemic regenerative potential. When evaluated alongside other biological age markers, it can help inform holistic treatment strategies.
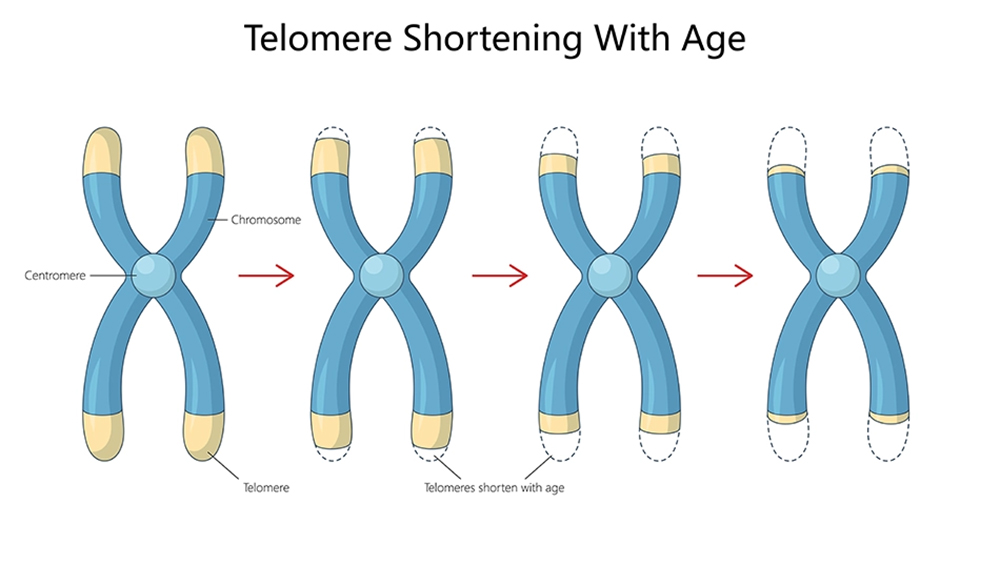
How Telomere Shortening Accelerates Visible Aging
Telomere erosion contributes significantly to skin aging and cellular dysfunction. As telomeres degrade, cells exhibit reduced mitotic capacity, which slows skin turnover, impairs collagen synthesis, and leads to signs like thinning skin, wrinkles, and reduced elasticity.
Environmental stressors, such as UV exposure, toxins, and psychological distress, accelerate this process. Telomere damage thus becomes a shared indicator of aesthetic aging and systemic decline. Maintaining telomere health in aesthetics is increasingly recognized as key to delaying visible signs of aging and improving treatment outcomes.
Slowing Down Telomere Shortening: What Actually Works
Reversing telomere shortening is not yet clinically feasible, but several strategies can help reduce its pace. These include prioritizing sleep, maintaining a balanced, antioxidant-rich diet, engaging in regular exercise, and managing chronic stress.
Bioactive compounds such as resveratrol, NAD+ precursors, TA-65, and senolytics are under active study for their cellular support roles. The epithalon peptide also shows potential in telomerase activation. These fall into the broader category of telomerase anti-aging therapy, though outcomes remain modest and preventive.
Telomere Testing in Aesthetic Practice: Value, Limits, and Application
Before integrating telomere-focused therapies, many clinics begin with telomere length testing to establish a baseline measure of biological aging. Most commonly, quantitative PCR (qPCR) is used due to accessibility, although more precise methods like Flow-FISH or Southern blotting are available in specialized labs. While these tests offer insight into systemic aging, results can vary depending on methodology, tissue type, and lab standards.
Clinicians should be cautious when interpreting telomere data. A single measurement offers limited predictive power, and it should always be evaluated alongside other biological age markers, lifestyle factors, and clinical context.
In aesthetic practice, telomere results can support decision-making for regenerative protocols, patient education, and long-term wellness planning, but should never be used as standalone diagnostic tools.
Clinical Use of Telomere Therapies in Aesthetic Medicine
As telomere science evolves, its relevance in aesthetic and longevity clinics continues to grow. Rather than attempting to lengthen telomeres outright, practitioners use indirect interventions to support cellular health:
- Telomere length testing helps establish biological age and customize treatment plans, though accuracy varies.
- NAD+ IV therapy is used to improve mitochondrial function and reduce oxidative stress, indirectly preserving telomere integrity.
- Peptides, including telomerase-activating options, are incorporated into multi-modal approaches. For a deeper understanding of how telomerase-activating peptides fit into aesthetic and anti-aging treatments, check out the Peptide Training course at HubMed Ed.
- Nutraceutical and antioxidant protocols, including polyphenols and adaptogens, help buffer against oxidative damage.
- Integrated aesthetic protocols that combine regenerative therapies with cellular aging support (e.g., PRP, stem cells) enhance long-term treatment resilience.
Framing these therapies as part of a preventive and regenerative strategy ensures patients understand their value without creating unrealistic expectations.
Adverse Effects and Risks of Telomere-Targeting Therapies
Growing excitement about telomere modulation requires a careful clinical perspective. While therapeutic activation of telomerase is intriguing, there are notable risks:
- Cancer potential: Telomerase is active in most cancers. Activating it improperly may increase oncogenic risk.
- Oversold products: Many supplements are unregulated and make exaggerated claims without data.
- Scientific uncertainty: Long-term safety, clinical protocols, and human efficacy are still being clarified.
- Unvetted candidates: Without screening, high-risk patients (e.g., with undiagnosed malignancy) may be exposed to danger.
Practitioners should avoid exaggerated promises, rely on evidence-based interventions, and communicate transparently with patients.
Cellular Mechanisms Behind Telomere Shortening and Senescence
Telomere attrition is primarily driven by the end-replication problem - DNA polymerase cannot fully replicate chromosome ends. External stressors like UV and pollution intensify this degradation.
Critically short telomeres activate cellular damage responses that halt division, pushing cells into senescence or apoptosis. Senescent cells release harmful cytokines and enzymes through SASP, leading to chronic inflammation and delayed tissue repair.
Who Is a Good Candidate for Telomere-Based Interventions?
Telomere-supportive treatments are best suited for patients showing signs of cellular stress or premature biological aging. Ideal candidates include:
- Patients with biological age exceeding chronological age, as indicated by functional assessments or testing.
- Individuals with poor recovery from aesthetic procedures or sluggish wound healing.
- Those with elevated oxidative stress, chronic fatigue, or lifestyle risk factors.
- Patients already focused on long-term wellness who will understand and commit to supportive protocols.
- Test-confirmed telomere shortening, where clinically relevant.
Ethical Concerns and the Future of Telomere Science in Aesthetics
As the line between longevity science and cosmetic enhancement blurs, ethical issues emerge. Should healthy individuals pursue telomere enhancement purely for appearance or perceived vitality?
Despite advances in animal studies, human trials remain limited. However, telomere research may one day support personalized aesthetic medicine by identifying ideal treatment windows and recovery profiles based on genetic aging metrics.
In Conclusion
Telomeres and aging offer a compelling view into the molecular foundations of aesthetic longevity. While current interventions remain largely supportive, their inclusion in treatment planning reflects a shift toward more preventive and biologically informed care.
To keep pace with innovation, explore VOD and masterclass aesthetic courses designed for professionals integrating cellular health, longevity protocols, and aesthetics. Telomere science isn’t a magic bullet, but when responsibly applied, it becomes a meaningful clinical asset.
FAQs
How to slow telomere shortening?
Telomere shortening can be slowed through lifestyle changes like reducing stress, eating antioxidant-rich foods, exercising, and getting quality sleep. Supplementation with NAD+, resveratrol, or peptides may offer modest benefits.
Are telomeres the key to aging and cancer?
Telomeres play a role in both aging and cancer. Shortened telomeres contribute to cellular aging, while excessive telomerase activity is linked to cancer progression.
What rebuilds telomeres?
Currently, no therapy fully rebuilds telomeres in humans. Some compounds may activate telomerase, which helps maintain telomere length, but this area needs more research.
What is the best supplement for telomeres?
Supplements like TA-65, epithalon peptide, NAD+ boosters, and resveratrol are being studied for their effects on telomeres. However, none have been definitively proven to elongate telomeres.
What is the strongest telomerase activator?
TA-65 and epithalon are among the most researched telomerase activators. However, their efficacy and long-term safety remain under investigation.
Does metformin lengthen telomeres?
Metformin may influence aging pathways but does not directly lengthen telomeres. It may offer protective effects through reduced oxidative stress and inflammation.
Do longer telomeres make you look younger?
Longer telomeres are associated with healthier cellular function and slower visible aging, but they are not a guarantee of youthful appearance. Other factors play a role in skin quality.
References:
- Schellnegger M, Hofmann E, Carnieletto M, Kamolz L-P. Unlocking longevity: the role of telomeres and their targeting interventions. Front Aging. 2024;5:1339317. doi:10.3389/fragi.2024.1339317. PMID: 38333665; PMCID: PMC10850353. https://pmc.ncbi.nlm.nih.gov/articles/PMC10850353/
- Shammas MA. Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care. 2011;14(1):28–34. doi:10.1097/MCO.0b013e32834121b1. PMID: 21102320; PMCID: PMC3370421. https://pmc.ncbi.nlm.nih.gov/articles/PMC3370421/
- Rossiello F, Jurk D, Passos JF, d’Adda di Fagagna F. Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol. 2022;24(2):135–147. doi:10.1038/s41556-021-00842-x. https://www.nature.com/articles/s41556-022-00842-x
- Gopenath TS, Shreshtha S, Basalingappa KM. Telomerase reactivation for anti-aging. In: Anti-Aging Drug Discovery on the Basis of Hallmarks of Aging. Academic Press; 2022:113–125. doi:10.1016/B978-0-323-90235-9.00005-7. https://www.sciencedirect.com/science/article/abs/pii/B9780323902359000057
Disclaimer:
This article is intended for licensed medical professionals. All protocols, dosages, and treatment insights referenced herein are based on published literature. The content is not intended to encourage application, diagnosis, or self-treatment of unlicensed individuals, and should not be used as a substitute for the clinical judgment of a qualified healthcare provider.

