
As interest in metabolic optimization grows, the use of targeted peptides such as the Cagrilintide peptide is gaining popularity in aesthetic and weight management circles. This long-acting amylin analog offers unique advantages over current therapies, particularly when used alongside GLP-1 receptor agonists. Its mechanism of action, dosing convenience, and synergy with semaglutide place it at the forefront of next-generation obesity pharmacotherapy.
Understanding the systemic support of metabolic peptides is becoming essential for aesthetic practitioners already investing in body contouring or facial harmonization. This evolving integration of metabolic and aesthetic therapies reflects the growing importance of the aesthetic courses for doctors at HubMed Ed, which support clinicians in staying at the forefront of peptide-based interventions.
Key Takeaways
- Cagrilintide is a long-acting amylin analog under investigation for weight loss.
- It works via the amylin and calcitonin receptors, enhancing satiety and reducing appetite.
- Clinical trials show substantial weight loss, especially when combined with semaglutide.
- It may offer a new solution for GLP-1-resistant patients.
- Still investigational: not FDA-approved, and patient counseling must reflect this.
What Is Cagrilintide?
Cagrilintide is a long-acting peptide engineered to mimic the function of amylin, a hormone normally released alongside insulin by pancreatic beta cells. Endogenous amylin supports appetite regulation and delays gastric emptying, contributing to glucose and energy balance.
However, its clinical utility is limited due to rapid enzymatic breakdown. This amylin analog peptide addresses this issue through molecular modifications that enhance stability and extend its half-life, allowing for convenient once-weekly subcutaneous administration in clinical protocols.
Currently under clinical investigation, cagrilintide is being evaluated both as a standalone treatment and in combination with semaglutide. Early research indicates significant promise for its dual role in appetite regulation and metabolic enhancement, particularly for patients unresponsive to GLP-1 monotherapy.
How Cagrilintide Works in the Body
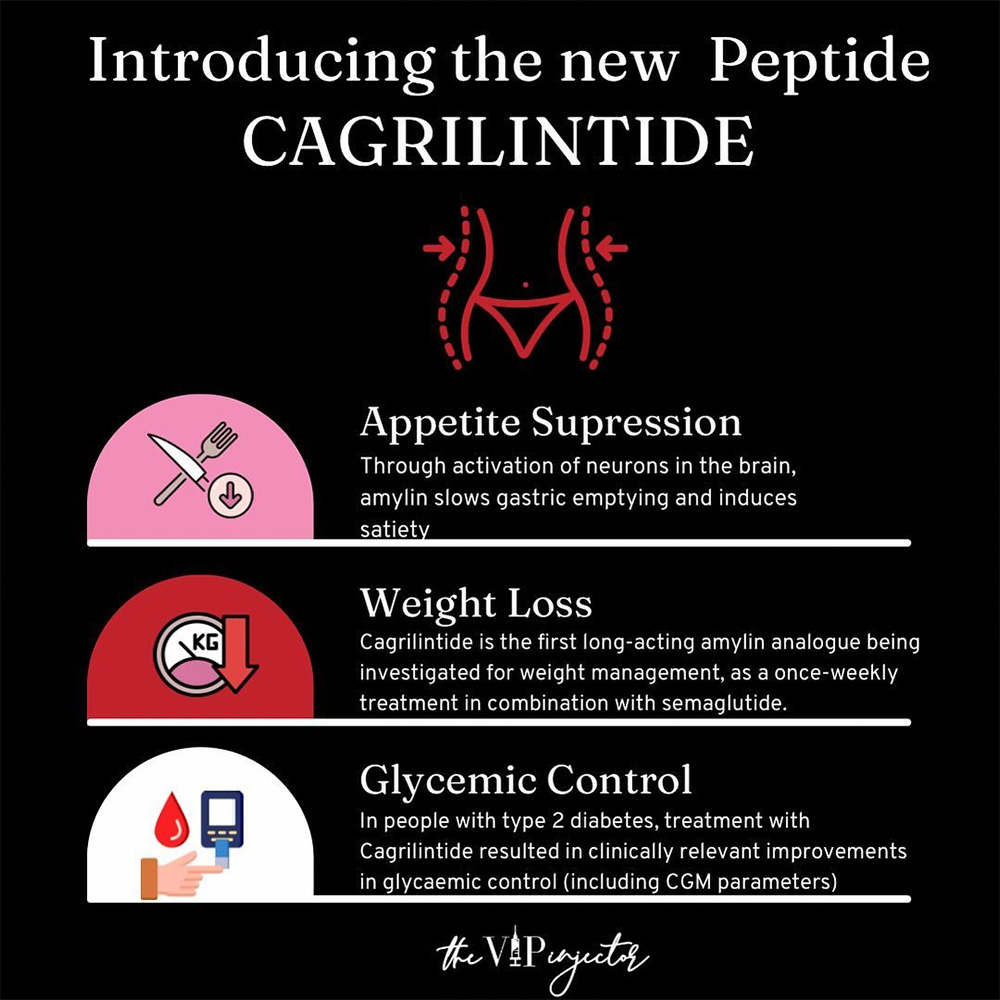
Unlike incretin-based therapies, cagrilintide acts primarily on amylin and calcitonin receptors in the brain. This interaction enhances satiety signaling, reducing hunger and promoting earlier meal termination. The prolonged gastric emptying further supports sustained postprandial fullness, a mechanism especially helpful for patients who struggle with frequent snacking or binge eating patterns.
Cagrilintide also shows an ability to reduce overall caloric intake and food cravings, particularly for high-fat or high-sugar foods. When paired with semaglutide, which targets GLP-1 receptors, the result is a complementary pathway approach, tapping into both incretin and amylin networks for broader metabolic control. This makes combination therapy particularly attractive in resistant cases or where long-term results are needed.
What Clinical Trials Tell Us
Cagrilintide clinical trials have shown promising outcomes in reducing appetite, improving adherence, and driving sustainable weight reduction.
Several clinical trials, including data published in The New England Journal of Medicine, have demonstrated that cagrilintide significantly reduces body weight in patients with obesity. When compared to placebo, patients receiving cagrilintide alone lost an average of 5-10% of their body weight. These effects were magnified in combination therapy protocols, with some studies noting weight reductions of up to 15% when paired with semaglutide.
Secondary endpoints in trials include reductions in waist circumference, visceral fat mass, appetite scores, and daily caloric intake. While cagrilintide is not yet FDA-approved, its ongoing development suggests strong potential as part of a new pharmacologic framework in obesity care.
Why Cagrilintide Could Change Obesity Care?
The mechanism behind Cagrilintide weight loss lies in its ability to enhance satiety and reduce meal frequency without the use of stimulants. Cagrilintide’s once-weekly dosing enhances patient adherence, especially when compared to daily or more frequent injectables.
Furthermore, cagrilintide peptide therapy offers an alternative for patients who plateau on GLP-1 monotherapy or cannot tolerate its full dosage. Its stimulant-free mechanism reduces the risk of anxiety or cardiovascular stress, making it suitable for a broader range of patient profiles, including those with type 2 diabetes or metabolic syndrome.

What Are the Side Effects of Cagrilintide?
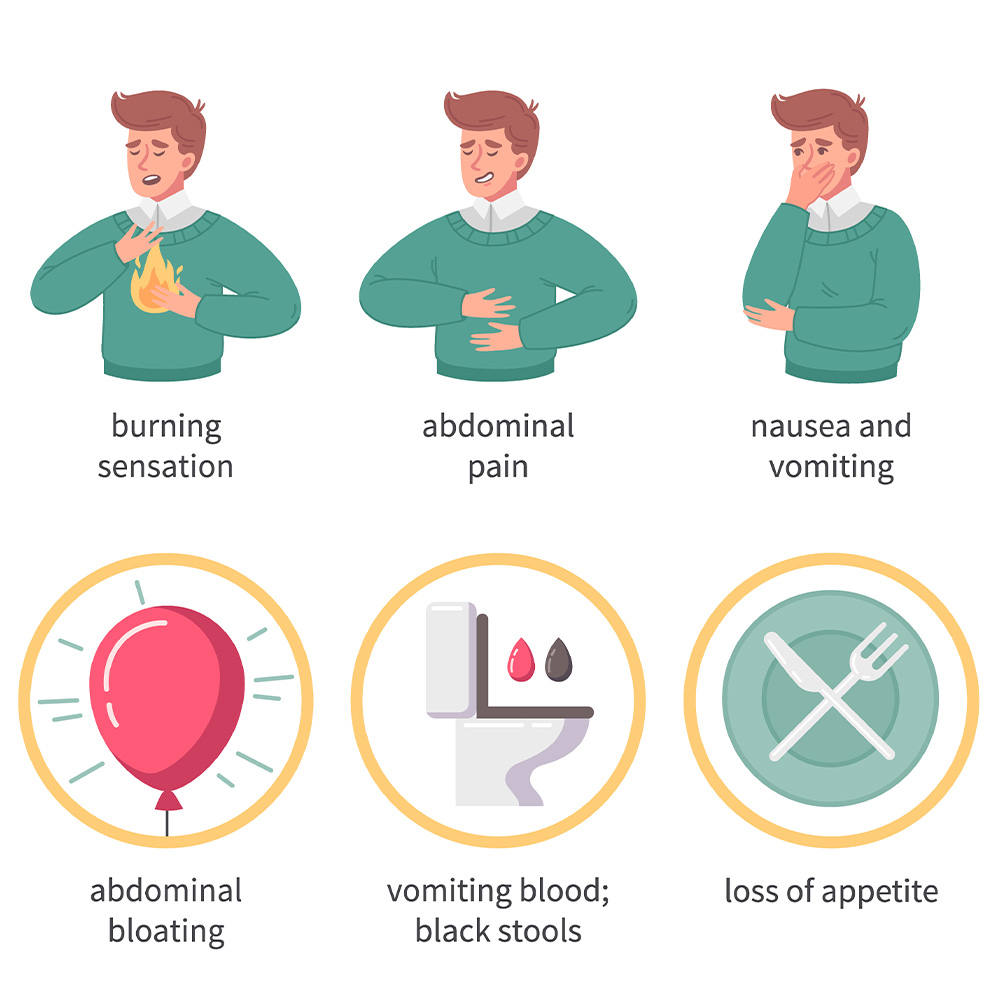
The safety profile of cagrilintide, as observed in clinical trials, is primarily characterized by gastrointestinal disturbances, most notably mild to moderate nausea during the initial titration phase. This effect is dose-dependent and typically transient, resolving as patients acclimate to therapy.
Reported side effects include:
- Nausea and queasiness, especially after meals during the first few weeks of dosing.
- Reduced appetite, which, while a therapeutic goal, may sometimes result in inadequate caloric intake if not monitored.
- Mild bloating or early satiety, likely related to delayed gastric emptying.
- Occasional vomiting, typically associated with rapid dose escalation.
These effects mirror what has been seen with GLP-1 therapies but are often less intense when appropriate titration protocols are followed. Importantly, the presence of side effects may also serve as a clinical cue that the appetite-regulating mechanism is active, though any severe or persistent symptoms warrant reevaluation of dose or co-therapy timing.
Cagrilintide Safety and Risks
As with any investigational peptide, cagrilintide should be used with clinical caution, particularly in populations with pre-existing endocrine or gastrointestinal disorders. While most adverse effects observed have been manageable, certain patient profiles present a higher risk for complications and should be screened carefully before treatment initiation.
Contraindications and clinical precautions include:
- Medullary thyroid carcinoma (MTC): Cagrilintide is contraindicated in individuals with a personal or family history of MTC due to its structural similarity to calcitonin, which may theoretically influence C-cell activity.
- Multiple endocrine neoplasia syndrome type 2 (MEN2): Patients with MEN2 should not receive cagrilintide because of its receptor overlap with calcitonin pathways.
- Severe gastrointestinal conditions: Those with delayed gastric emptying, gastroparesis, or chronic GI dysmotility may experience exacerbated symptoms due to the drug’s action on gastric motility.
- Pregnancy and breastfeeding: Cagrilintide has not been evaluated in pregnant or lactating individuals and should be avoided unless clinically justified.
- Severe renal impairment (eGFR <30 mL/min/1.73 m²): Use with caution due to insufficient safety data and possible increased risk of adverse events in this population.
Although cardiovascular risk has not been elevated in short-term trials, comprehensive long-term data are still pending. Until full safety data are available, cautious patient selection and baseline risk assessment are advised.
Cagrilintide vs. Semaglutide: Key Differences and Synergies
The comparison of Cagrilintide vs semaglutide highlights how dual-pathway modulation may offer more robust results than single-agent approaches alone.
While semaglutide remains the cornerstone of GLP-1-based weight management, cagrilintide introduces a new dimension by activating amylin receptors. Their mechanisms differ, but their goals overlap: appetite suppression, reduced energy intake, and improved glycemic regulation.
Clinical trials show enhanced outcomes when cagrilintide and semaglutide are combined. Patients not only experience more weight loss but also improved satiety and fewer cravings. Practitioners should monitor overlapping side effects like nausea and titrate doses accordingly. For a deeper look into Semaglutide mechanism and clinical use, practitioners can refer to the What Is Semaglutide article at HubMed Ed.
What the Future Holds for Cagrilintide
Ongoing studies are assessing long-term cardiovascular outcomes, weight loss durability, and real-world adherence. As research expands, we may see cagrilintide incorporated into combination protocols not just with semaglutide, but potentially tirzepatide, retatrutide, and other emerging incretin-mimetics.
If approved, cagrilintide could become a first-line option in pharmacologic obesity management, especially within aesthetic settings where facial fullness, contouring, and body composition improvements are key patient goals.
In Conclusion
Cagrilintide is an emerging peptide therapy that may soon redefine weight loss support in both clinical and aesthetic settings. Its unique mechanism and synergy with GLP-1 agonists position it as a powerful future tool in the hands of trained professionals. While still investigational, the preliminary data are encouraging for those specializing in metabolic and cosmetic outcomes.
Practitioners interested in the evolving role of peptides in aesthetic medicine are encouraged to explore the Peptide Therapy Training at HubMed Ed for deeper clinical insight and patient protocol design. Feel free to browse through our previous masterclass courses.
FAQs
Is Cagrilintide better than tirzepatide?
These two peptides work via different pathways. While tirzepatide is a dual GLP-1/GIP agonist, cagrilintide acts via the amylin system. Ongoing trials are comparing outcomes.
How much weight can you lose on Cagrilintide?
In clinical trials, participants lost between 5% and 15% of body weight, especially when combined with semaglutide. Results vary by patient profile and dosage.
Does Cagrilintide slow gastric emptying?
Yes, one of its primary mechanisms is delayed gastric emptying, which enhances feelings of fullness and reduces meal frequency.
How long does Cagrilintide stay in your system?
Cagrilintide has a long half-life, supporting once-weekly subcutaneous dosing. This increases patient convenience and treatment adherence.
Can Cagrilintide be taken alone?
Yes, it is being studied both as monotherapy and in combination with GLP-1 drugs. However, greater weight loss has been observed when used together with semaglutide.
References:
- Davies MJ, Bajaj HS, Broholm C, et al; for the REDEFINE 2 Study Group. Cagrilintide–Semaglutide in Adults with Overweight or Obesity and Type 2 Diabetes. N Engl J Med. 2025;393(7):648–659. doi:10.1056/NEJMoa2502082. https://www.nejm.org/doi/full/10.1056/NEJMoa2502082
- Frias JP, Deenadayalan S, et al. Efficacy and safety of co-administered once-weekly cagrilintide 2–4 mg with once-weekly semaglutide 2–4 mg in type 2 diabetes: a multicentre, randomised, double-blind, active-controlled, phase 2 trial. Lancet. 2023;402(10403):720–730. doi:10.1016/S0140-6736(23)01461-2. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)01163-7/abstract
- Garvey WT, Blüher M, Contreras CO, Davies MJ, Lehmann EW, Pietiläinen KH, et al. Coadministered Cagrilintide and Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2025;393(7):635–647. doi:10.1056/NEJMoa2502081. https://www.nejm.org/doi/full/10.1056/NEJMoa2502081
- D’Ascanio AM, Mullally JA, Frishman WH. Cagrilintide: A Long-Acting Amylin Analog for the Treatment of Obesity. Cardiol Rev. 2024 Jan-Feb;32(1):83–90. doi:10.1097/CRD.0000000000000513. Epub 2023 Oct 20. https://pubmed.ncbi.nlm.nih.gov/36883831/
Disclaimer:
This article is intended for licensed medical professionals. All protocols, dosages, and treatment insights referenced herein are based on published literature. The content is not intended to encourage application, diagnosis, or self-treatment of unlicensed individuals, and should not be used as a substitute for the clinical judgment of a qualified healthcare provider.

