Some patients heal and respond well to treatment, others don’t, even with the same technique. In many cases, the difference isn’t the procedure itself but the patient’s underlying biology. One key factor is cellular senescence - the buildup of aged, inactive cells that no longer divide but still affect surrounding tissue.
Understanding how senescence affects skin regeneration can help improve both treatment planning and results. The regenerative medicine course offered by HubMed Ed dives deeper into the role of cellular aging in aesthetic outcomes, giving clinicians practical tools to apply this science in daily practice.
What Is Cellular Senescence?
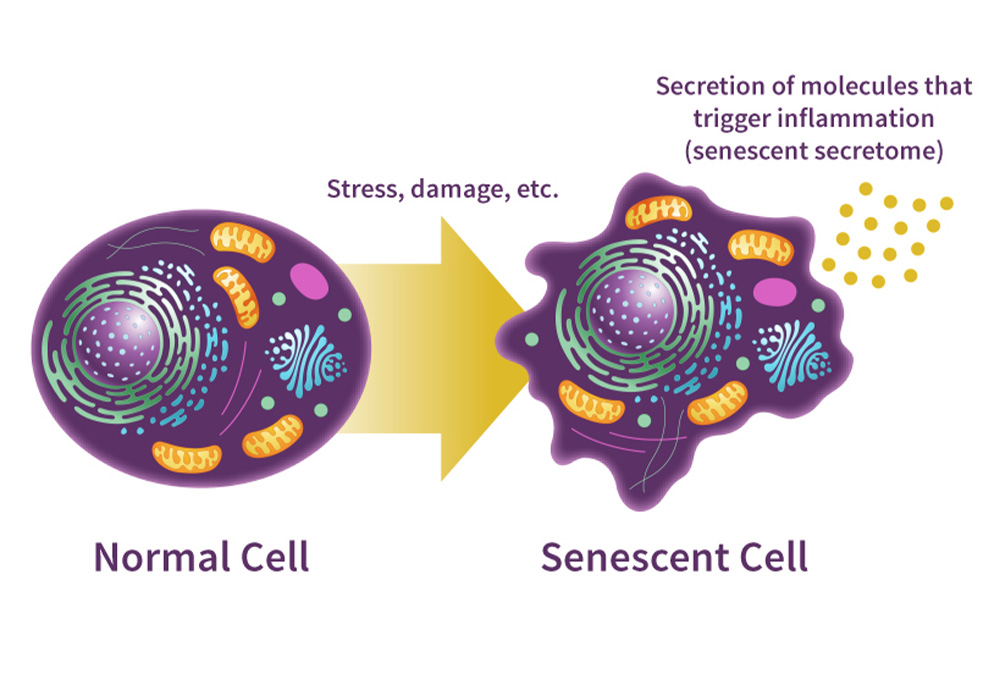
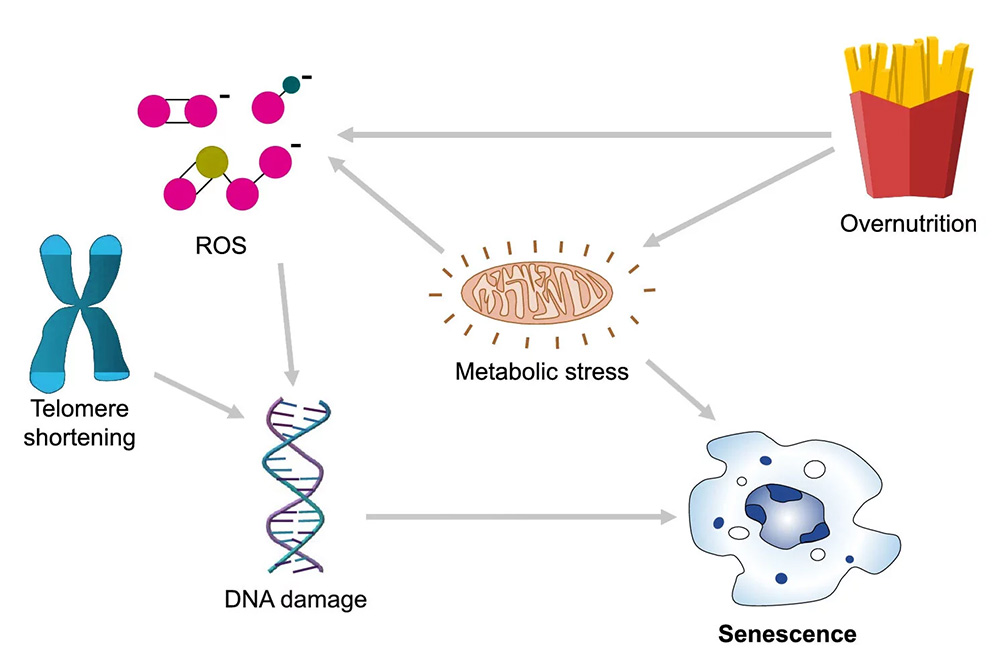
Cellular senescence is a biological state in which a cell permanently exits the cell cycle but remains metabolically active. This process typically occurs in response to DNA damage, oxidative stress, or telomere shortening and serves as a defense mechanism against malignant transformation. In short-term contexts, it helps prevent cancer and aids in wound healing.
However, senescent cells do not simply disappear. They accumulate and begin to secrete a cocktail of pro-inflammatory cytokines, enzymes, and growth factors known as the senescence-associated secretory phenotype (SASP). This secretion can disrupt local tissue architecture, stimulate nearby cells to also become senescent, and degrade extracellular matrix components. While protective initially, chronic senescence becomes a driver of visible aging and systemic dysfunction.
Connection Between Senescent Cells and Systemic Aging
Senescence is not limited to the skin, as it impacts nearly every organ system. As senescent cells accumulate throughout the body, they contribute to a condition called inflammaging, characterized by chronic low-grade inflammation. This systemic inflammation weakens immune responses, impairs metabolic efficiency, and increases the risk of age-related diseases.
In aesthetic patients, especially those experiencing accelerated or premature aging, the presence of senescent cells may be a contributing factor. These cells can lead to more pronounced fatigue, poor tissue healing, and diminished skin vitality. Such systemic effects emphasize the importance of assessing biological aging beyond surface appearance when planning aesthetic treatments.
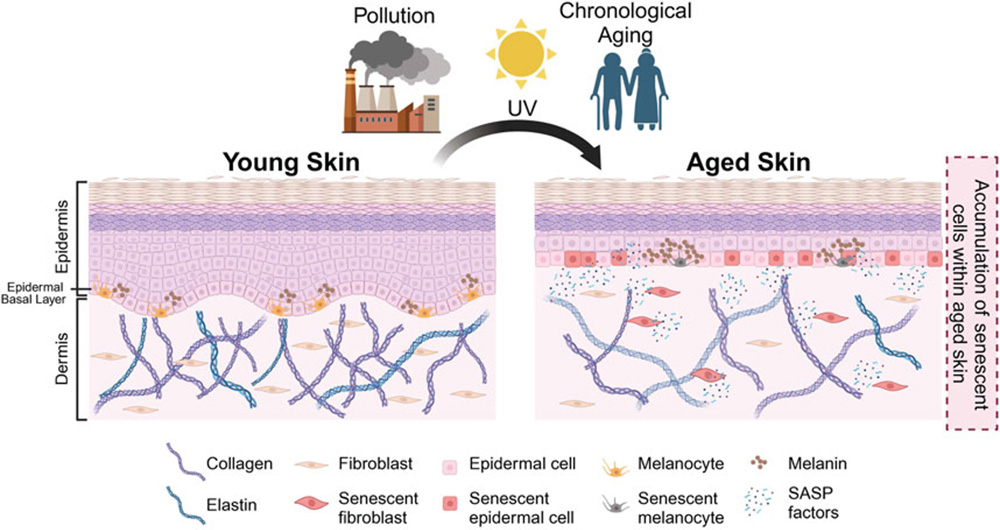
Senescent Cell Accumulation and Skin Aging Mechanisms
In the skin, senescence manifests primarily through the dysfunction of fibroblasts and keratinocytes. Senescent fibroblasts lose their ability to synthesize collagen and elastin effectively, resulting in decreased dermal density and elasticity. Keratinocytes in a senescent state compromise the epidermal barrier and slow turnover, leading to dullness and increased susceptibility to irritation.
Furthermore, SASP factors, also known as pro-aging secretions, exacerbate pigmentation irregularities and stimulate melanogenesis, contributing to uneven tone and age spots. These secretions also impair skin recovery, which explains why mature skin doesn’t heal as well in procedures or under environmental stress. This direct relationship between senescent cells and aging helps clarify many of the clinical signs of bio-aged skin.
The Impact of Senescence on Aesthetic Medicine Outcomes
When patients don’t heal as expected or show muted responses to regenerative therapies, senescence may be the underlying reason. Aging cells respond poorly to reparative cues, leading to sluggish tissue turnover, weakened matrix remodeling, and flat post-procedure outcomes.
Treatments like microneedling, PRP, lasers, and radiofrequency rely on a responsive cellular environment. In skin burdened with senescent cells, the expected cascade of regeneration often stalls. Recognizing this barrier allows practitioners to adjust protocols, set realistic expectations, and consider layering adjunctive biologic support.
Patients with high senescent cell burden may also show inconsistent or minimal improvement from dermal fillers, as the extracellular matrix (ECM) becomes degraded and less able to support volumization. Incorporating senescence profiling into patient evaluation can help set realistic expectations, select ideal candidates, and personalize protocols for improved satisfaction.
Therapeutic Strategies for Targeting Senescent Cells
Emerging interventions now aim to clear or modulate senescent cells, offering new tools for aesthetic rejuvenation. Senolytics, such as quercetin, fisetin, and dasatinib, actively eliminate senescent cells, while senomorphics suppress SASP factors without inducing cell death. Some peptide-based topicals and antioxidant blends are also showing promise in reducing cellular senescence in skin.
These agents help normalize cellular signaling while calming the inflammatory environment driven by aging cells. As interest grows, senolytics for skin rejuvenation are being explored for their potential to selectively eliminate aging cells and improve dermal function. Additionally, delivery options vary. Oral, injectable, and topical routes are being explored, though most remain off-label in aesthetic practice.
Clinical Integration and Treatment Planning
Incorporating senescence awareness into practice starts with identifying potential indicators: slow wound healing, poor treatment response, and visible signs of bio-aging in younger patients. Clinicians should use these cues to adjust protocols, layering senescence-modulating agents with energy-based treatments or integrating cellular support like NAD+ or exosomes.
Patient education plays a key role. Communicating the concept of biological aging versus chronological age helps patients understand treatment limitations and sets the stage for a long-term rejuvenation strategy. Also, tools like skin imaging, diagnostic assessments, and patient questionnaires can help support a more personalized, integrative approach.
Potential Risks and Safety Considerations in Senescence Modulation
Not all senescent cells are harmful. Some are essential for tissue remodeling and immune signaling. Indiscriminate elimination of senescent cells can disrupt beneficial processes, such as wound healing or tumor suppression.
Senolytics, particularly when used systemically, may cause off-target effects, especially if dosed improperly. Releasing SASP factors during clearance can trigger acute inflammation, which may overwhelm a patient with impaired detoxification capacity. Extra caution is needed for patients with autoimmune disorders, liver impairment, or chronic stress. These cases require thorough consent, clear explanation of risks, and careful monitoring when incorporating senescence-targeting therapies into aesthetic treatments.
Senescence, NAD+, and Mitochondrial Health
Senescent cells often exhibit mitochondrial dysfunction, and this is closely tied to declining NAD+ levels. NAD+ is vital for DNA repair, oxidative balance, and activation of sirtuins, key enzymes involved in longevity and cellular stress response.
By restoring NAD+ through injections or IV therapy, clinicians may support cellular resilience, reduce oxidative stress, and enhance repair functions. Addressing mitochondrial health through NAD+ may also enhance strategies that target senescence and aesthetics in synergy. This is particularly relevant for older aesthetic patients or those undergoing intensive treatments. NAD+ supplementation, while not a direct senolytic, can delay senescence onset and enhance restorative function.

Identifying Ideal Patients for Senescence-Modulating Treatments
Senescence-targeting interventions are not universally appropriate. Recognizing which patients are most likely to benefit helps improve outcomes and ensures ethical application of these emerging therapies. Look for the following profiles when assessing candidacy:
- Patients showing signs of biological aging, such as chronic low-grade inflammation, slow healing, or diminished skin vitality.
- Those who have a poor response to traditional treatments or present with generalized skin fatigue.
- Individuals with high stress levels, disrupted sleep, or systemic imbalances that accelerate cellular aging.
- Lifestyle factors such as poor diet, sedentary behavior, and smoking can also signal increased senescent cell burden.
- Ideal patients are often motivated to address the root causes of aging and are open to longer-term treatment strategies.
It’s important to be transparent when discussing off-label or emerging therapies to ensure patients can make informed choices. Evaluate candidates based on biological rather than just chronological age. Furthermore, always take time for thorough screening, consent, and honest communication about what the treatment can and can’t achieve.
Emerging Innovations in Senescence-Targeted Aesthetics
Recent advances are expanding the possibilities for precision-based senescence targeting in aesthetic medicine. These technologies promise to improve both efficacy and safety by refining how, where, and when senescent cells are addressed. Innovations to watch include:
- Exosome-based delivery systems capable of transporting senolytics directly into target tissues with high specificity.
- AI-powered senescence mapping tools that may soon allow clinicians to visualize areas of cellular aging and tailor treatment strategies accordingly.
- Nanocarrier technologies designed to improve the penetration and cellular uptake of active ingredients aimed at senescent cell clearance.
- Smart peptides and designer molecules engineered to suppress SASP or promote selective apoptosis of senescent cells.
- Integrated protocols combining senescence therapies with PRP, stem cells, and regenerative injectables to enhance multi-layered rejuvenation outcomes.
As these tools develop, senescence is expected to become a foundational concept in biologically personalized aesthetics.
The Bottom Line
Cellular senescence isn’t just an academic concept, but also a clinical reality that directly affects the results. From inconsistent healing to weakened tissue structure, senescent cell buildup can sabotage even the best-designed aesthetic protocols. By addressing these cells through targeted strategies, clinicians can better match treatment with tissue biology.
To explore how these insights apply in practice, join VOD and live aesthetic courses covering senescence science, NAD+ support, and regenerative pairing techniques. It’s time to move beyond the surface and treat aging where it begins.
FAQs
What is the difference between aging and senescence?
Aging refers to the gradual decline of physiological function over time. Senescence is a specific cellular process where cells stop dividing but remain metabolically active, often contributing to aging.
What is an example of a senescence?
Skin fibroblasts exposed to UV light may become senescent, stopping division and secreting inflammatory molecules that degrade collagen and elastin.
What are the first signs of senescence?
Early signs include slowed healing, increased skin dullness, loss of elasticity, and visible fine lines even with minimal environmental damage.
Which vitamin is anti-senescent?
Vitamin D and Vitamin C have shown anti-senescent properties, especially in skin. They help modulate oxidative stress and support healthy cell cycles.
What are the diseases associated with senescence?
Senescent cells are linked to Alzheimer’s, type 2 diabetes, osteoarthritis, cardiovascular disease, and certain cancers due to chronic inflammation.
Which hormone promotes senescence?
Persistent high cortisol levels, often from chronic stress, can accelerate cellular senescence. Estrogen decline in menopause also contributes to skin senescence.
How to get rid of senescent cells naturally?
Strategies include intermittent fasting, exercise, a polyphenol-rich diet (e.g., quercetin, fisetin), and reducing oxidative stress through antioxidants.
References:
- Cellular senescence. In: Complementary Therapies in Medicine. ScienceDirect; 2016. Accessed July 15, 2025. https://www.sciencedirect.com/topics/medicine-and-dentistry/senescence
- Qin Y, Liu H, Wu H. Cellular senescence in health, disease, and lens aging. Pharmaceuticals. 2025;18(2):244. doi:10.3390/ph18020244. https://www.mdpi.com/1424-8247/18/2/244
- Witham MD, Granic A, Miwa S, et al. New horizons in cellular senescence for clinicians. Age Ageing. 2023;52(7):afad127. doi:10.1093/ageing/afad127. https://pmc.ncbi.nlm.nih.gov/articles/PMC10355181/
- Mansfield L, Ramponi V, Gupta K, et al. Emerging insights in senescence: pathways from preclinical models to therapeutic innovations. npj Aging. 2024;10:53. doi:10.1038/s41514-024-00152-w. https://www.nature.com/articles/s41514-024-00181-1
- McHugh D, Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol. 2018;217(1):65-77. doi:10.1083/jcb.201708092. https://pmc.ncbi.nlm.nih.gov/articles/PMC5748990/
- Konstantinou E, Longange E, Kaya G. Mechanisms of senescence and anti-senescence strategies in the skin. Biology. 2024;13(9):647. doi:10.3390/biology13090647. https://www.mdpi.com/2079-7737/13/9/647
Disclaimer:
This article is intended for licensed medical professionals. All protocols, dosages, and treatment insights referenced herein are based on published literature. The content is not intended to encourage application, diagnosis, or self-treatment of unlicensed individuals, and should not be used as a substitute for the clinical judgment of a qualified healthcare provider.
Disclaimer:
This article is intended for licensed medical professionals. All protocols, dosages, and treatment insights referenced herein are based on published literature. The content is not intended to encourage application, diagnosis, or self-treatment of unlicensed individuals, and should not be used as a substitute for the clinical judgment of a qualified healthcare provider.