
Age reversal is no longer science fiction. Breakthroughs in epigenetic reprogramming are uncovering ways to reset cellular aging by modifying gene expression. Rather than treating surface-level symptoms, aesthetic professionals can now target the underlying mechanisms that drive aging.
This approach opens the door to true tissue rejuvenation, restoring function, not just appearance. Backed by emerging science, epigenetic reprogramming may shift aesthetic medicine from cosmetic enhancement to long-term regenerative care. As research progresses, it could redefine how we approach longevity and skin health.
What Is Epigenetic Reprogramming?
Epigenetics refers to modifications in gene expression without altering the underlying DNA sequence. These modifications, mainly DNA methylation and histone acetylation, serve as biological switches that turn genes on or off based on environmental stimuli, aging, and internal signaling.
Epigenetic reprogramming aims to reset these gene expression patterns to a more youthful state. By reversing the accumulated “epigenetic noise” that causes cellular dysfunction, this process seeks to restore optimal cellular behavior. In aesthetic and regenerative medicine, this opens new avenues for long-term revitalization and biological resilience. This scientific approach is now being investigated across various clinical domains as a potential way to reverse aging with epigenetics rather than merely treating its visible signs.

Biological vs. Chronological Aging: The Role of the Epigenetic Clock
Chronological age reflects time, but biological age reflects the physiological state of our tissues. The discrepancy between the two explains why some individuals appear younger or older than their years. The epigenetic clock, pioneered by Dr. Steve Horvath, measures biological age through specific DNA methylation markers.
Known as the Horvath Clock, this model analyzes methylation at specific CpG sites across the genome to estimate an individual’s true biological age. It offers a molecular-level snapshot of aging progression. By using these clocks, clinicians can track how lifestyle, disease, and interventions affect aging on a cellular level. Targeting epigenetic aging allows aesthetic providers to develop more predictive and personalized treatment protocols that go beyond surface-level improvements.
Yamanaka Factors and the Mechanics of Reprogramming
The discovery of the Yamanaka factors: Oct4, Sox2, Klf4, and c-Myc, revolutionized our understanding of cell plasticity. When introduced into adult cells, these transcription factors can reprogram them into induced pluripotent stem cells (iPSCs), effectively erasing their epigenetic age.
This discovery laid the foundation for Yamanaka factors anti-aging research, where cellular reprogramming is used not to create stem cells, but to rewind aging markers while preserving cell identity.
However, full reprogramming carries the risk of tumorigenesis, the formation of tumors due to uncontrolled cell growth, resulting from complete cellular dedifferentiation. Partial reprogramming, which involves transient activation of these factors, retains cellular identity while promoting youthful gene expression. This carefully managed process is at the core of modern age-reversal research.
Preclinical Evidence: Reversing Aging in Animal Models
Studies in mice have demonstrated that intermittent expression of Yamanaka factors can reinvigorate tissue function, regenerate nerves, improve cardiac performance, and even reverse vision loss. These findings suggest that reprogramming doesn’t just halt aging - it may undo it.
Nonetheless, animal models have limitations. Differences in lifespan, physiology, and genetic complexity mean that we must approach human application with cautious optimism. Human clinical trials remain in the exploratory phase, with safety and scalability being primary challenges.
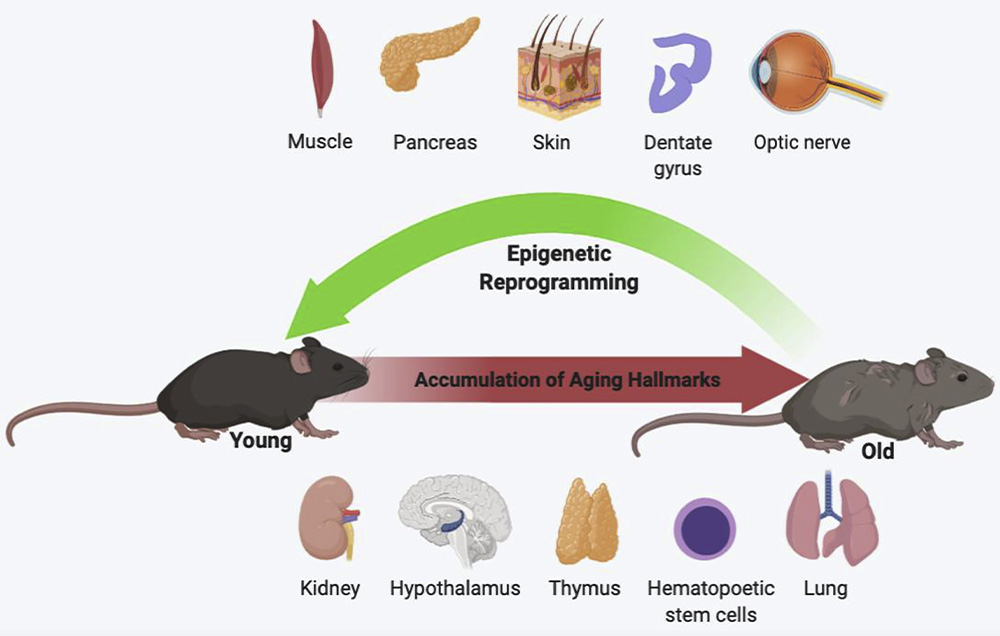
Clinical Promise in Aesthetic and Regenerative Medicine
Epigenetic reprogramming may improve skin texture, elasticity, and vascular function by targeting aging pathways in fibroblasts, keratinocytes, and endothelial cells. This shift elevates aesthetic practice from superficial enhancements to preventive and regenerative care.
Incorporating biomarkers of epigenetic aging into aesthetic assessments could transform treatment planning and patient outcomes. As research progresses, cellular reprogramming for skin is becoming a core concept in next-generation aesthetic protocols focused on long-term dermal health and visible revitalization.
Combining Epigenetic Reprogramming with Regenerative Therapies
As regenerative aesthetics continues to evolve, combining epigenetic reprogramming with established biologic therapies offers a powerful next step in treatment innovation. Aesthetic professionals exploring these intersections through a regenerative medicine course will be better equipped to harness this synergy for long-term, cellular-level revitalization.
Here are key therapies where epigenetic reprogramming shows the most promising potential for integration and enhanced clinical effect:
- PRP (Platelet-Rich Plasma): Reprogramming may improve fibroblast responsiveness to growth factors, enhancing skin repair and longevity of results.
- Exosome therapy: Rejuvenated cells demonstrate heightened receptivity to exosomal signals, potentially increasing their regenerative impact.
- Stem cell therapies: Epigenetic optimization of stem cells can boost their survival, integration, and differentiation in targeted aesthetic applications.
- Microneedling with biologics: When combined with reprogramming agents, microneedling may deliver deeper and more effective dermal renewal.
- Fat grafting and SVF: Enhancing the regenerative quality of adipose-derived cells could result in better graft retention and reduced inflammation.
- Energy-based devices: Reprogramming may improve post-treatment recovery and amplify tissue remodeling when used alongside RF or laser procedures.
Technologies and Challenges in Delivering Reprogramming Agents
Delivering Yamanaka factors or similar epigenetic modulators into the body requires precision and control. Current experimental methods include viral vectors, mRNA injections, peptide-based approaches, and topical nanoformulations. Each of these technologies faces distinct challenges, from immune reactions to instability and inefficient cellular uptake.
The clinical challenge lies in ensuring safety, specificity, and reproducibility. Overexpression or imprecise delivery can lead to undesirable outcomes like tumorigenesis or loss of tissue identity. Scalable, targeted, and regulated protocols remain a high research priority as the practice moves toward clinical application.
Risks, Limitations, and Ethical Considerations in Clinical Use
Despite its promise, epigenetic reprogramming carries substantial risks. Introducing pluripotency factors increases the risk of teratoma formation, especially if reprogramming is uncontrolled. There’s also the potential for disrupting essential gene expression pathways, particularly when delivery systems are not tightly regulated.
Ethically, offering age reversal to healthy individuals raises concerns about equity, informed consent, long-term safety, and psychological well-being. The aesthetic setting introduces additional complexity, as patient expectations must be carefully managed to prevent misuse or overpromising results. Transparency, caution, and data-driven protocols will be key to ethically navigating this emerging space.
Regulatory Landscape and the Future of Human Application
Epigenetic reprogramming currently remains in the realm of preclinical and early translational research. While the science is rapidly advancing, there are no approved therapeutic protocols for aesthetic use in humans at this time. Given the complexity and potential risks of gene editing and cellular manipulation, regulatory agencies are taking a cautious, tightly controlled approach.
Nonetheless, the accessibility of epigenetic testing and longevity-focused interventions is on the rise. As the field matures, aesthetic professionals need to stay informed, critically evaluate emerging tools, and advocate for ethical, evidence-based integration.
Personalizing Protocols Based on Epigenetic Biomarkers
Tools like the Horvath Clock allow clinicians to assess a patient’s epigenetic age and monitor treatment impact over time. Emerging tests measure DNA methylation patterns in blood, saliva, or skin biopsies to guide personalized anti-aging protocols.
This biomarker-driven approach aligns with precision medicine goals. Key advantages include:
- Objective age assessment: Tools like the Horvath Clock analyze CpG methylation to provide an accurate biological age metric.
- Non-invasive testing: Many tests use blood or saliva, making them patient-friendly and easy to repeat over time.
- Tailored protocols: Clinicians can adapt treatment plans based on methylation status, adjusting frequency, modality, or regenerative support.
- Outcome monitoring: Repeated tests can track the efficacy of epigenetic or aesthetic interventions at a molecular level.
In Conclusion
Epigenetic reprogramming represents one of the most exciting frontiers in anti-aging science, with the potential to fundamentally reset the biological age of cells. It signifies a shift toward regenerative, longevity-driven care. As the science evolves, the key to responsible application lies in education and collaboration.
Explore ongoing developments through aesthetic courses at HubMed Ed, including longevity science updates, gene-targeted therapy modules, and future-focused clinical roundtables. Embracing this next wave of aesthetic medicine will empower practitioners to lead in the era of biologically informed revitalization.

FAQs
What are the epigenetic signs of aging?
Epigenetic signs include increased DNA methylation in certain genes, histone modifications, and chromatin remodeling that silence youthful gene expression and activate inflammatory pathways.
Can epigenetic aging be reversed?
Emerging evidence suggests that epigenetic age can be partially reversed through interventions like caloric restriction, exercise, and experimental reprogramming using Yamanaka factors.
What is the Harvard study of reverse aging?
The 2023 Harvard study showed that loss of epigenetic information, not DNA mutations, may drive aging and that restoring this information in mice reversed age-related damage.
Is epigenetics legitimate?
Yes. Epigenetics is a well-established scientific field with decades of research showing how gene expression is modified by external and internal factors without changing DNA.
How do I find out my epigenetic age?
You can test your epigenetic age using commercial DNA methylation tests or through research-grade assays like the Horvath Clock.
Can gene therapy reverse ageing?
Gene therapy may hold promise for age reversal, particularly through modulation of epigenetic regulators, though it remains experimental and not yet approved for human use.
Who is the billionaire trying to reverse aging?
Bryan Johnson, founder of Blueprint, is one of several high-profile individuals investing heavily in age reversal technologies and personal longevity protocols.
References:
- Pereira B, Correia FP, Alves IA, et al. Epigenetic reprogramming as a key to reverse ageing and increase longevity. Ageing Res Rev. 2024;95:102204. doi:10.1016/j.arr.2024.102204. https://pubmed.ncbi.nlm.nih.gov/38272265/
- Yücel AD, Gladyshev VN. The long and winding road of reprogramming-induced rejuvenation. Nat Commun. 2024;15:1941. doi:10.1038/s41467-024-42474-3. https://www.nature.com/articles/s41467-024-46020-5
- Panchin AY, Ogmen A, Blagodatski SA, et al. Targeting multiple hallmarks of mammalian aging with combinations of interventions. Aging (Albany NY). 2024;16(16):12073–12100. doi:10.18632/aging.206078. https://www.aging-us.com/article/206078
- Yang JH, Petty CA, Dixon-McDougall T, et al. Chemically induced reprogramming to reverse cellular aging. Aging (Albany NY). 2023;15(13):5966-5989. doi:10.18632/aging.204896. https://pmc.ncbi.nlm.nih.gov/articles/PMC10373966/
- Cipriano A, Moqri M, Maybury-Lewis SY, Rogers-Hammond R, et al. Mechanisms, pathways, and strategies for rejuvenation through epigenetic reprogramming. Nat Aging. 2023;4(1). doi:10.1038/s43587-023-00539-2. https://www.researchgate.net/publication/376568296_Mechanisms_pathways_and_strategies_for_rejuvenation_through_epigenetic_reprogramming
- Simpson DJ, Olova NN, Chandra T. Cellular reprogramming and epigenetic rejuvenation. Clin Epigenetics. 2021;13:170. doi:10.1186/s13148-021-01144-6. https://clinicalepigeneticsjournal.biomedcentral.com/articles/10.1186/s13148-021-01158-7
- Salk Institute. Turning back time: Salk scientists reverse signs of aging. Published December 15, 2016. Accessed July 14, 2025. https://www.salk.edu/news-release/turning-back-time-salk-scientists-reverse-signs-of-aging/
- Ocampo A, Reddy P, Martinez-Redondo P, et al. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell. 2016;167(7):1719-1733.e12. doi:10.1016/j.cell.2016.11.052. https://www.cell.com/cell/fulltext/S0092-8674(16)31664-6
- Dutchen S. Loss of epigenetic information can drive aging, restoration can reverse it. Harvard Medical School News. Published January 12, 2023. Accessed July 14, 2025. https://hms.harvard.edu/news/loss-epigenetic-information-can-drive-aging-restoration-can-reverse-it
Disclaimer:
This article is intended for licensed medical professionals. All protocols, dosages, and treatment insights referenced herein are based on published literature. The content is not intended to encourage application, diagnosis, or self-treatment of unlicensed individuals, and should not be used as a substitute for the clinical judgment of a qualified healthcare provider.
Disclaimer:
This article is intended for licensed medical professionals. All protocols, dosages, and treatment insights referenced herein are based on published literature. The content is not intended to encourage application, diagnosis, or self-treatment of unlicensed individuals, and should not be used as a substitute for the clinical judgment of a qualified healthcare provider.

