Non-surgical rejuvenation continues to evolve fast. Mesotherapy and dermal fillers remain cornerstone treatments in aesthetic medicine, but 2025 has brought notable shifts in the sector: an increased focus on skin quality (skin-boosters), more conservative, natural results, and a growing emphasis on safety and evidence-based practice.
Mesotherapy and Dermal Filler Fundamentals for Modern Practice
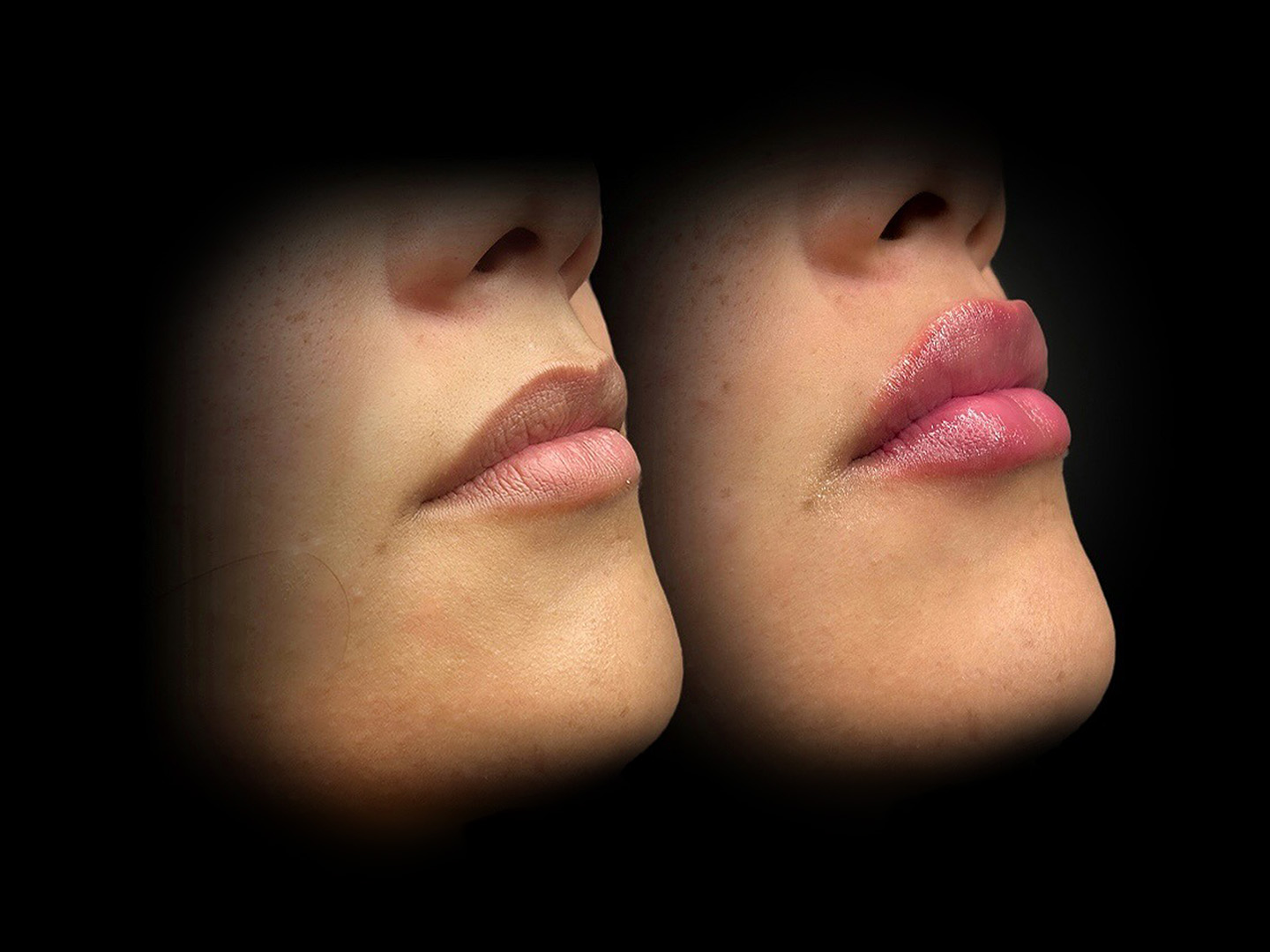
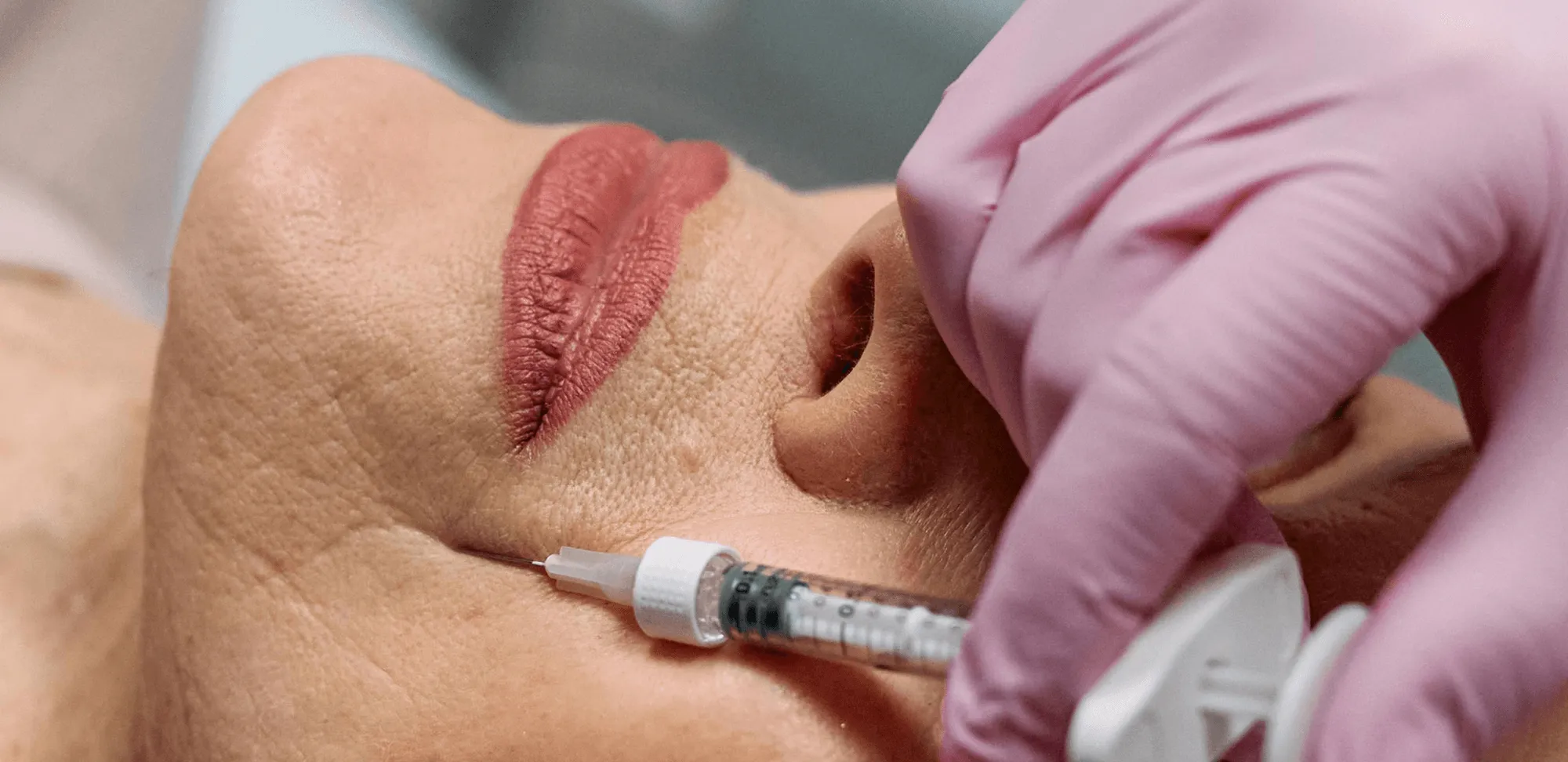
Let’s start by defining mesotherapy. Mesotherapy delivers micro-injections of active substances into the dermis to improve hydration, stimulate collagen, and address localized concerns like under-eye hollows, skin laxity, and small fat deposits. Originating with Dr. Michel Pistor in 1952, the technique has been adapted into many formulations and delivery styles. Dermal fillers (most commonly hyaluronic acid — HA) restore volume, smooth lines, and sculpt facial contours. Newer biostimulatory products (e.g., CaHA, PLLA) encourage collagen formation for longer-term improvement.
2025 Clinical Trends Shaping Mesotherapy Treatment and Dermal Filler Techniques
Skin Quality Before Volume
Skin quality first: “skin-boosters” are on the rise. Practitioners are prioritizing skin texture and hydration over dramatic volumizing. Micro-droplet HA skin-boosters — designed to improve skin smoothness and glow — have moved from Europe into the U.S. market, with FDA-cleared products now available like Kybella and others only to be applied with microneedling, like PromoItalia. This shift reflects growing patient demand for subtle, natural results rather than large volume changes.
The Shift Toward Natural Aesthetic Results
Measured, natural results beat “more is better.” There’s a clear cultural pivot toward refinement and restoration (sometimes called “deflation” or natural aesthetic trends). Many patients and practitioners now prefer modest enhancements; some are even choosing to dissolve previous overfilled areas to return to a softer, more natural look.
Technique and Safety Are Essential
Technique and safety matter more than ever. As procedures become more mainstream, regulators and professional societies emphasize safety: correct anatomy, cannula use, aspiration practices, and immediate access to emergency protocols (e.g., hyaluronidase). The American Society of Plastic Surgeons and other authorities underscore that many mesotherapy mixtures are not FDA-approved as formulated, so practitioners must exercise caution and fully inform patients of the risks.
Risks of Unregulated Mesotherapy Devices and At-Home Injectables
Beware of unregulated shortcuts. Non-medical devices and at-home injectables (e.g., hyaluron pens, dubious “fat-dissolving” cocktails) have attracted FDA warnings due to safety incidents. These tools often bypass important safeguards and can cause serious outcomes (infection, occlusion, scarring). Licensed providers should avoid them and educate patients about the risks.

Best Practices for Safe Mesotherapy and Dermal Filler Procedures
- Choose products backed by data: Favor FDA-cleared skin-boosters/fillers or well-studied formulations and stay current with manufacturer guidance and peer-reviewed literature. (For example, early FDA approvals for micro-droplet HA products have helped standardize a previously unregulated niche.)
- Refine technique: Microcannulas, layered placement, conservative boluses, and careful injection depth produce better and safer aesthetic outcomes. Invest in hands-on training and rehearsal under experienced supervision.
- Document and consent meticulously: Record product brand, lot number, and a clear emergency transfer plan. Explicitly document that certain mesotherapy mixes may be off-label and discuss realistic expectations and risks with the patients.
- Prioritize skin health: Combine injectable approaches with skin care, topical agents, and energy-based devices where appropriate for synergistic results.

Safety Protocols and FDA Guidelines for Mesotherapy and Dermal Filler Treatments
Mesotherapy caveat: Many mesotherapy cocktails (unique mixes of vitamins, enzymes, and other agents) are compounded and may not be FDA-approved as combined injectable products. Professional societies advise transparency with patients and careful adherence to sterile technique and appropriate clinical indications.
FDA and skin-boosters: The arrival of regulated micro-droplet HA products is a positive step; it provides standardized formulations for a treatment that historically varied widely by clinic. Still, practitioners must follow approved indications and administration instructions.
Bottom line Mesotherapy and dermal fillers remain powerful tools for aesthetic medicine, but the emphasis is shifting: quality of skin, conservative volume, and safety are the priorities. For clinicians, the path forward is clear—invest in high-quality products, continuous hands-on training, and rigorous consent/documentation to protect patients and practices.
Join us: If you’re a clinician seeking practical, up-to-date training in mesotherapy and fillers, consider in-person supervised programs (like our Fort Lauderdale mesotherapy and dermal fillers workshop taking place next 11 October ) that combine evidence-based theory with real-world hands-on experience under Sarmiento’s supervision.

Sources (key references)
- Grand View Research — Skin Boosters Market Report (2024–2030). 
- FDA – SKINVIVE by Juvéderm (micro-droplet HA skin booster approval).
- American Society of Plastic Surgeons – Position on Mesotherapy/Injection Lipolysis.
- People / industry coverage — trend toward dissolving fillers and natural looks (2024–2025).
- FDA / public warnings on at-home injectables and hyaluron pens (safety concerns).
- This article and all other paid articles on the platform
- Discounts for video classes and live Masterclasses
- Exclusive podcasts, roundtables, and webinars